Our Research
A major focus of my research is to study the neural and immunological mechanisms of neuropsychiatric disorders such as depression and anxiety. We use a combination of transgenic mice, immune cell transplantation, optogenetics/electrophysiology, viral mediated gene transfer, behavioral models and molecular methods to understand how the brain and body adapts to stress to control pathological behaviors in depression and anxiety.
Projects
Circuit mechanisms of stress-induced social deficits.
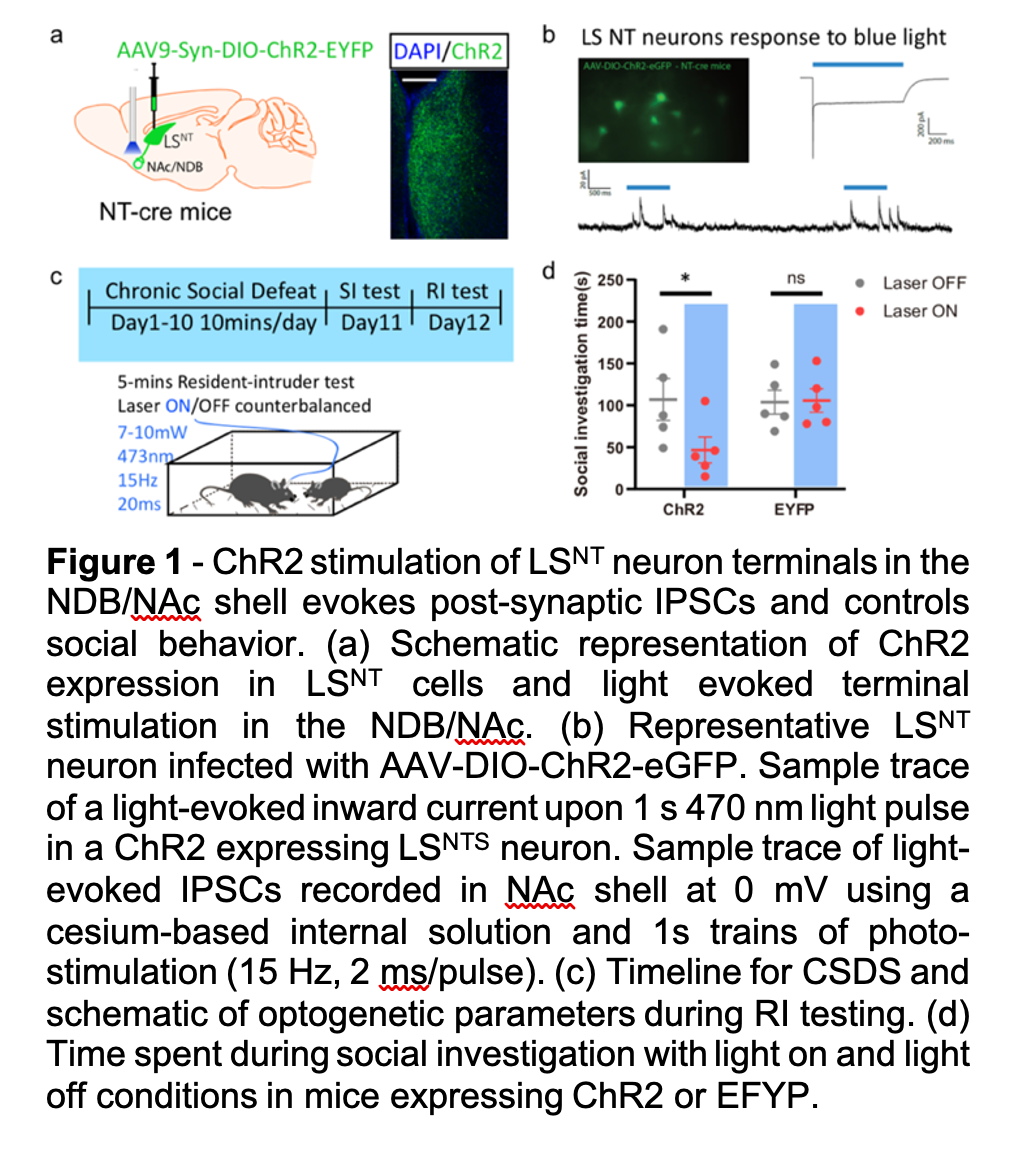 Social avoidance manifests across a host of psychiatric illnesses with causes ranging from disinterest in social contact to negative emotional states evoked by social encounters. While the causes of social avoidance are diverse, past social trauma can result in severe social avoidance thought to reflect reduced social reward. Despite a deep clinical understanding of social trauma and its resultant effects on social behavior, we know very little about the underlying neural circuitry involved. Preclinical social trauma models have been utilized in recent years to better understand the biological underpinnings of such changes in social behavior. For example, the CSDS model has been utilized extensively to understand how reward circuitry mediates social interaction behavior, yet very little is known regarding the underlying meaning of such behavioral deficits. In the Russo lab we’ve taken a multipronged approached by assessing molecular, cellular, and physiological alterations throughout the brain that mediate susceptibility to social stress as defined by long lasting social avoidance and reduced responses to natural rewards. In one of our recent projects, we utilized an unbiased iDisco+ approach coupled with advanced tracing methods to identify the lateral septum (LS) and its downstream targets as being highly activated in both male and female susceptible mice during avoidance of a normally rewarding social target. Functional studies with optogenetics and chemogenetics show that activation of the LS elicits social avoidance and prevents context dependent social reward by inhibiting downstream reward centers (Figure 1). Our work is providing fundamentally new insight into the neural mechanisms underlying post-stress social reward deficits.
Social avoidance manifests across a host of psychiatric illnesses with causes ranging from disinterest in social contact to negative emotional states evoked by social encounters. While the causes of social avoidance are diverse, past social trauma can result in severe social avoidance thought to reflect reduced social reward. Despite a deep clinical understanding of social trauma and its resultant effects on social behavior, we know very little about the underlying neural circuitry involved. Preclinical social trauma models have been utilized in recent years to better understand the biological underpinnings of such changes in social behavior. For example, the CSDS model has been utilized extensively to understand how reward circuitry mediates social interaction behavior, yet very little is known regarding the underlying meaning of such behavioral deficits. In the Russo lab we’ve taken a multipronged approached by assessing molecular, cellular, and physiological alterations throughout the brain that mediate susceptibility to social stress as defined by long lasting social avoidance and reduced responses to natural rewards. In one of our recent projects, we utilized an unbiased iDisco+ approach coupled with advanced tracing methods to identify the lateral septum (LS) and its downstream targets as being highly activated in both male and female susceptible mice during avoidance of a normally rewarding social target. Functional studies with optogenetics and chemogenetics show that activation of the LS elicits social avoidance and prevents context dependent social reward by inhibiting downstream reward centers (Figure 1). Our work is providing fundamentally new insight into the neural mechanisms underlying post-stress social reward deficits.
Neuroimmune mechanisms of stress and depression.
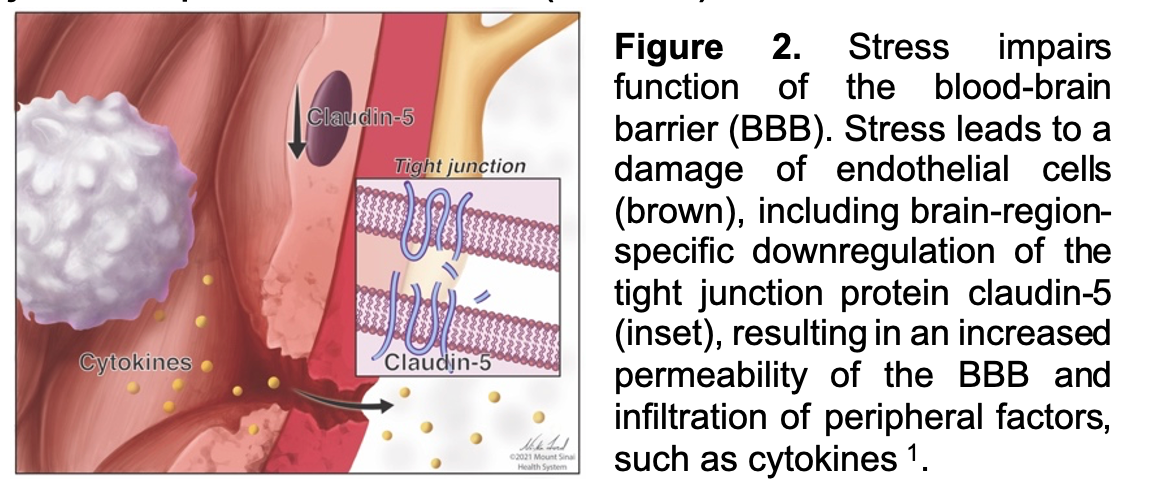 Work from our group and others suggests that elevated peripheral immune factors, which can gain access to limbic regions of brain via stress-related breakdown of the BBB (Figure 2), may represent an important factor driving behavioral symptoms in depression. In a seminal study, we have shown that chronic social defeat stress (CSDS) in mice results in loss of the tight junction protein Claudin-5 (CLDN5) and breakdown of the endothelial barrier allowing greater entry of IL-6 directly into brain reward regions like the NAc and mPFC—although the latter is sex specific and only occurs in female mice. Similar changes in CLDN5 mRNA in postmortem human brain from MDD subjects has also been observed, providing us with early evidence to link human MDD with BBB impairments. In terms of specific symptom domains and constructs, we and others have shown that there is a strong association between the immune system and reward dysfunction, and that increased peripheral inflammation correlates with clinical measures of anhedonia. However, the fundamental mechanisms by which the peripheral immune system and BBB interact ultimately leading to behavioral changes is not well understood. Using a computational approach to filter existing bulk RNA-seq data to identify endothelial-enriched transcripts, we found many genes differentially expressed between MDD and healthy control (HC) subjects. We also found that in MDD subjects increased BBB-permeability correlates with increased anhedonia. This is further substantiated by the finding that opening of the BBB by viral-mediated downregulation of CLND5 in NAc of mice increases vulnerability to stress-induced reward dysfunction. While the pathophysiology of increased BBB permeability is not yet known, we are using rodent models, informed by clinical data, to provide an increasingly sophisticated understanding of the mechanisms facilitating immune-brain interactions in depression.
Work from our group and others suggests that elevated peripheral immune factors, which can gain access to limbic regions of brain via stress-related breakdown of the BBB (Figure 2), may represent an important factor driving behavioral symptoms in depression. In a seminal study, we have shown that chronic social defeat stress (CSDS) in mice results in loss of the tight junction protein Claudin-5 (CLDN5) and breakdown of the endothelial barrier allowing greater entry of IL-6 directly into brain reward regions like the NAc and mPFC—although the latter is sex specific and only occurs in female mice. Similar changes in CLDN5 mRNA in postmortem human brain from MDD subjects has also been observed, providing us with early evidence to link human MDD with BBB impairments. In terms of specific symptom domains and constructs, we and others have shown that there is a strong association between the immune system and reward dysfunction, and that increased peripheral inflammation correlates with clinical measures of anhedonia. However, the fundamental mechanisms by which the peripheral immune system and BBB interact ultimately leading to behavioral changes is not well understood. Using a computational approach to filter existing bulk RNA-seq data to identify endothelial-enriched transcripts, we found many genes differentially expressed between MDD and healthy control (HC) subjects. We also found that in MDD subjects increased BBB-permeability correlates with increased anhedonia. This is further substantiated by the finding that opening of the BBB by viral-mediated downregulation of CLND5 in NAc of mice increases vulnerability to stress-induced reward dysfunction. While the pathophysiology of increased BBB permeability is not yet known, we are using rodent models, informed by clinical data, to provide an increasingly sophisticated understanding of the mechanisms facilitating immune-brain interactions in depression.
Immune biomarkers in depression.
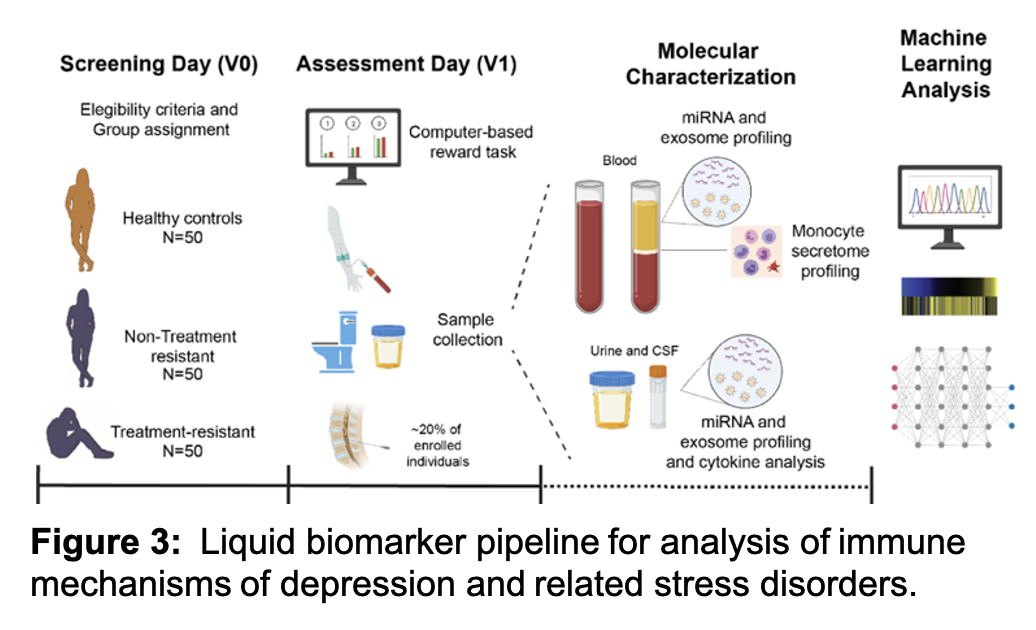 Our lab works closely with clinical parterns to identify liquid biomarkers associated with unipolar depression, post traumatic stress disorders (PTSD) and neuropsychiatric post-acute sequelae of SARS CoV-2 (pasc) infection. The overall objective of the work is to provide a rigorous characterization of body fluid-based (e.g., ‘liquid’) biosignatures of stress signatures in humans (Figure 3). Based on our ongoing and published work in rodent stress models and in humans with depression and anhedonia, we have identified three highly promising categories of liquid biosignatures: (1) inflammatory monocyte-derived factors, (2) microRNAs (miRNAs), and (3) extracellular vesicles (EVs), also known as exosomes. We use information gathered from experiments assessing each of these 3 biosignatures and use predictive modeling to predict symptoms, such as deficits on effort-based reward discounting and other behavioral measures of anhedonia (‘external features’). We are also building an integrated model based on the predictors identified, thereby moving the field closer to developing a multi-channel liquid biosignature for stress-related disorders. Since the work reflects translation into humans from completed and ongoing behavioral and molecular neuroscience studies in rodent stress models, our translational research program is aiming to define the biological basis of depression and PTSD.
Our lab works closely with clinical parterns to identify liquid biomarkers associated with unipolar depression, post traumatic stress disorders (PTSD) and neuropsychiatric post-acute sequelae of SARS CoV-2 (pasc) infection. The overall objective of the work is to provide a rigorous characterization of body fluid-based (e.g., ‘liquid’) biosignatures of stress signatures in humans (Figure 3). Based on our ongoing and published work in rodent stress models and in humans with depression and anhedonia, we have identified three highly promising categories of liquid biosignatures: (1) inflammatory monocyte-derived factors, (2) microRNAs (miRNAs), and (3) extracellular vesicles (EVs), also known as exosomes. We use information gathered from experiments assessing each of these 3 biosignatures and use predictive modeling to predict symptoms, such as deficits on effort-based reward discounting and other behavioral measures of anhedonia (‘external features’). We are also building an integrated model based on the predictors identified, thereby moving the field closer to developing a multi-channel liquid biosignature for stress-related disorders. Since the work reflects translation into humans from completed and ongoing behavioral and molecular neuroscience studies in rodent stress models, our translational research program is aiming to define the biological basis of depression and PTSD.
Neural Circuit Mechanisms of Aggression.
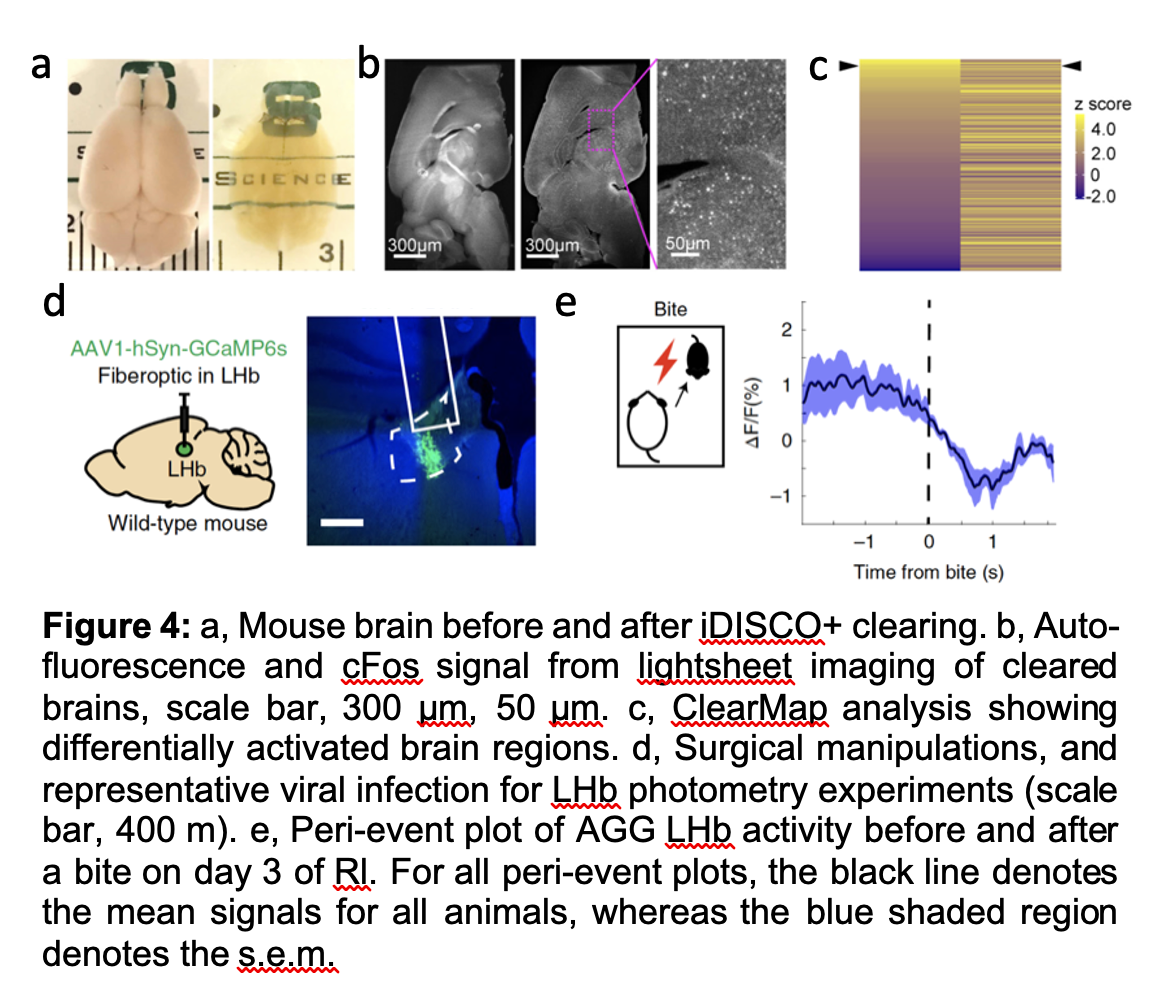 Aggression is a collection of evolutionarily conserved behaviors meant to control social hierarchies and protects valuable resources like mates, food, and territory. In most cases, aggression is a necessary, adaptive component of social behavior. However, aggression towards others can be considered pathological when it threatens lives, increases risk of depression and anxiety in victims and witnesses, and incurs major economic burdens on society. While such aggressive behavior can manifest across a wide range of psychiatric and neurological diseases, there are few, if any, approved treatments aimed specifically at curbing it. Despite the massive costs of violence on society, the pervasiveness of aggression among psychiatric patients, and our current lack of treatments, aggression has been understudied, particularly in females, relative to other emotional behaviors. A growing number of preclinical studies in recent years has shed some light on a limited number of brain regions, cell types, and neural ensembles governing specific components of aggressive behavior, however, there remains significant gaps in our understanding. We take a broad approach at understanding the neural circuit mechanisms governing aggressive social encounters. By Using behavior analysis, whole brain cFos analysis, computational biology, electrophysiology and in vivo Ca2+ imaging, we have shown that nuclei within the ventral midbrain can encode and directly modulate the motivational or rewarding component of aggressive vs. non-aggressive social behavior in both males and females (Figure 4). We are currently extending upon this work in many important ways, including an unbiased network analysis in which we hope to identify sex specific neural circuit control over aggressive social encounters.
Aggression is a collection of evolutionarily conserved behaviors meant to control social hierarchies and protects valuable resources like mates, food, and territory. In most cases, aggression is a necessary, adaptive component of social behavior. However, aggression towards others can be considered pathological when it threatens lives, increases risk of depression and anxiety in victims and witnesses, and incurs major economic burdens on society. While such aggressive behavior can manifest across a wide range of psychiatric and neurological diseases, there are few, if any, approved treatments aimed specifically at curbing it. Despite the massive costs of violence on society, the pervasiveness of aggression among psychiatric patients, and our current lack of treatments, aggression has been understudied, particularly in females, relative to other emotional behaviors. A growing number of preclinical studies in recent years has shed some light on a limited number of brain regions, cell types, and neural ensembles governing specific components of aggressive behavior, however, there remains significant gaps in our understanding. We take a broad approach at understanding the neural circuit mechanisms governing aggressive social encounters. By Using behavior analysis, whole brain cFos analysis, computational biology, electrophysiology and in vivo Ca2+ imaging, we have shown that nuclei within the ventral midbrain can encode and directly modulate the motivational or rewarding component of aggressive vs. non-aggressive social behavior in both males and females (Figure 4). We are currently extending upon this work in many important ways, including an unbiased network analysis in which we hope to identify sex specific neural circuit control over aggressive social encounters.
Russo Laboratory
Scott J Russo, PhD
Endowed Chair, Mount Sinai Professor in Affective Neuroscience
Director, Center for Affective Neuroscience
Director, Brain-Body Research Institute
Location
Lab: ICAHN ICAHN 10-02
Office: ICAHN 10-20c
Phone
Office: 212.659.5917
Lab: 212.659.8625
Email: scott.russo@mssm.edu
Featured Publications
Meet the Team

Johana Alvarez
PhD Student

Rachel Fisher
PhD Student

Hsiao-yun Lin
Instructor

Hyoseon Oh
Postdoctoral Fellow

Romain Durand
Instructor

Antonio Aubry
Instructor

Eva Kuzyk
PhD Student

Adelina Grusca
PhD Student

Xuanming Guo
Postdoctoral Fellow

Sarah Colaiuta
Research Technician

Teagan Daly
Master Student

Hamilton Oh
Postdoctoral Fellow

Yanmin Luo
Visiting Scientist

Huipeng Huang
Visiting Researcher
Alumni

Hossein Aleyasin
Postdoctoral Fellow

Georgia Hodes
Postdoctoral Fellow

Daniel Christoffel
PhD Student

Samer Muhareb
Volunteer

Aki Takahashi, Ph.D.
Visiting Assistant Professor

Tory Drescher
MS Student

Jia-Ru Chung
Master Student

Anna Brancato
Visiting PHD student

Yusuke Shimo
Postdoctoral Fellow

Charles J. Burnett
Postdoctoral Fellow

Flurin Cathomas
Postdoctoral Fellow

Long Li
Instructor

Mitra Heshmati
MD, PhD Student

Madeline Pfau
PhD Student

Kristina Deonaraine
Master Student

Meghan Flanigan
PhD Student

Ajani Gyasi
MS Student

Lyonna Parise
Instructor

Manuella Pinto-Kaster
Visiting Assistant Professor

Sam Golden
PhD Student

Caroline Menard
Postdoctoral Fellow

Katherine LeClair
PhD Student

Elizabeth Karpman
Research Coordinator

Kenny Chan
Instructor
Funding & Awards
1R01MH104559-01 (Russo PI) 06/01/2014-05/30/2019 NIMH Peripheral IL-6 from leukocytes controls susceptibility to social defeat stress. The goal of this award is to understand the molecular mechanisms within leukocytes that control susceptibility versus resilience to social stress.
1P50AT008661-01 (Russo PI for project 1) 7/01/2015-6/30/2020 NCAM Project 1: Preservation of psychological resilience The goal of this grant is to test the mechanisms by which grape derived polyphenol extracts promote psychological resilience.
1R01 MH090264 (Russo PI) 11/01/2015-10/31/2020 NIMH Role of thalamic versus cortical inputs to nucleus accumbens in stress related disorders The goal of this award is to define the electrophysiological and behavioral effects of thalamic versus cortical glutamate inputs to the NAc in the chronic social defeat stress model.
Irma T. Hirschl/Monique Weill-Caulier Trust Research Award (Russo PI) 01/01/2014-12/302019 Leukocyte derived IL-6 predicts individual differences in susceptibility to social defeat stress The goal of this award is to understand whether IL-6 from leukocytes controls the pro-depressant effects of chronic social defeat stress.
2002 NIDA CPDD Junior Investigator Travel Award
2003 CUNY Robert L. Thompson Award for Excellence
2005 Postdoctoral Ruth L. Kirschstein National Research Service Award (NIH)
2006 Keystone Symposia Scholarship Award
2006 Grand Prize; University of Texas Southwestern Medical Center Postdoctoral Symposium
2006 NARSAD Young Investigator Award
2007 Invited travel award for NIDA mini-convention at SFN
2008 NARSAD Young Investigator Award
2009 National Academy of Science Kavli Frontiers Fellow
2012 Johnson & Johnson/IMHRO Rising Star Translational Research Award
2012 Dr. Harold and Golden Lamport Research Award
2012 Mount Sinai School of Medicine “Best Postdoctoral Mentor” Award
2013 Irma T. Hirschl/Monique Weill-Caulier Trust Research Award
2014 Icahn School of Medicine at Mount Sinai Faculty Council Award
2015 Thomson Reuters “Highly Cited Researcher”
2015 Thomson Reuters “Most Influential Scientific Minds”
2017 Neuroscience Mentorship Distinction Award
2022 Neuroscience Mentorship Distinction Award
2022: Travel Award, Society of Biological Psychiatry (Flurin Cathomas)
2024 – 2025: Postdoctoral Research Abroad Fellowship, National Research Foundation of Korea (Hyoseon Oh)
2023 – 2025: K99/R00 Pathway to Independence Award, National Institute on Drug Abuse (Tony Aubry)
2023 – 2025: K99/R00 Pathway to Independence Award, National Institute of Diabetes and Digestive and Kidney Diseases (Kenny Chan)
2023: Doft Family Postdoctoral Innovator Award, Friedman Brain Institute (Tony Aubry)
2022 – 2024: NARSAD Young Investigator Grant, Brain and Behavior Research Foundation (Romain Durand-de Cuttoli)
2022 – 2024: NARSAD Young Investigator Grant, Brain and Behavior Research Foundation (Lyonna Parise)
2022 – 2024: NARSAD Young Investigator Grant, Brain and Behavior Research Foundation (Kenny Chan)
2021 – 2023: NARSAD Young Investigator Grant, Brain and Behavior Research Foundation (Long Li)
2020 – 2022: NARSAD Young Investigator Grant, Brain and Behavior Research Foundation (Flurin Cathomas)
2019 – 2021: Leon Levy Fellowship, Leon Levy Foundation (Lyonna Parise)
2019 – 2022: CIHR Postdoctoral Fellowship, Canadian Institutes of Health Research (Kenny Chan)
2017: Postdoctoral Fellowship, Kurt and Senta Hermann Foundation (Flurin Cathomas)
2017-2019 Early Postdoc Mobility Fellowship, Swiss National Science Foundation; Walter and Gertrud Siegenthaler Postdoctoral Fellowship (Flurin Cathomas PI)
2017-2019 F31 Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NRSA), National Institute of Mental Health (Meghan Flanigan PI)
2017-2019 Brain and Behavior Research Foundation Young Investigator Grant (Caroline Menard PI)
2017 Recruitment of science and technology personnel, Ministry of Science and Technology Grand in Taiwan, China Medical University (Hsiao-yun Lin PI)
2014-2016 F31 Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NRSA), National Institute of Mental Health (Madeline Pfau PI)
2013-2016 F30 Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NRSA), National Institute of Mental Health (Mitra Heshmati PI)
2012-2014 Brain and Behavior Research Foundation Young Investigator Grant (Georgia Hodes PI)
2025: Travel Award, Alzheimer’s Association International Conference (Hamilton Oh)
2024: Master of Science in Biomedical Science Award for Excellence in Leadership and Service, Icahn School of Medicine at Mount Sinai (Tory Drescher)
2024: Fellow, Summer Program in Neuroscience, Excellence and Success (SPINES) (Johana Alvarez)
2024: Travel Award, Society for Advancement of Chicanos/Hispanics & Native Americans in Science (Johana Alvarez)
2024: NSP Associate, Neuroscience Scholars Program (Johana Alvarez)
2024: NSP Associate, Neuroscience Scholars Program (Rachel Fisher-Foye)
2024: Best Poster, Friedman Brain Institute Neuroscience Retreat (Rachel Fisher-Foye)
2024: Travel Award, Society for Neuroeconomics (Romain Durand-de Cuttoli)
2024: 1st Place Presentation, XVIIIth Little Brain Big Brain (Kenny Chan)
2023: Travel Award, Winter Conference on Brain Research (Rachel Fisher-Foye)
2023: Trainee Professional Development Award, Society for Neuroscience (Rachel Fisher-Foye)
2023: Best Poster, Friedman Brain Institute Neuroscience Retreat (Rachel Fisher-Foye)
2023: Best Paper, Society for Neuroeconomics (Romain Durand-de Cuttoli)
2023: Travel Award, The National Hispanic Science Network (Lyonna Parise)
2022: FENS/IBRO-PERC Travel Award, Federation of European Neuroscience Societies (Romain Durand-de Cuttoli)
2020: Postdoctoral Award, Philippe Foundation (Romain Durand-de Cuttoli)
2019: Travel Award, Center for Neurotechnology and Behavior (Tony Aubry)
2018: Best Record of Achievement in Neuroscience (BRAIN), Friedman Brain Institute (Katherine LeClair)
2017: Society for Neuroscience (SfN) travel award for Japan Neuroscience Society meeting, Chiba (Japan) (Meghan Flanigan)
2016: Society for Neuroscience (SfN) travel award for FENS meeting, Copenhagen (Denmark) (Meghan Flanigan)
2016: American College of Neuropsychopharmacology (ACNP) travel award for ACNP meeting, Hollywood (USA) (Caroline Menard)
2015: Best Record of Achievement in Neuroscience (BRAIN), Friedman Brain Institute (Meghan Flanigan)
Media
 Medical News Today
Medical News Today
Neuroscience of bullying: Why do some find it rewarding?
 Medical Express
Medical Express
Stress-related inflammation may increase risk for depression
 Psychology Today
Psychology Today
A Bully’s Brain Perceives Subordinating Others as a “Reward”
 ScienceNews
ScienceNews
His stress is not like her stress
 Nature
Nature
Nature Podcast
 News Medical Life Sciences
News Medical Life Sciences
Study reveals that people with stress-related inflammation may suffer from depression
One Mind Institute
Inflammation and Depression: Translating Basic Discoveries into New Therapeutics – Dr. Scott Russo
![]() Business Standard
Business Standard
How stress ups depression risk
 Wisconsin Public Radio
Wisconsin Public Radio
New Research Shows Bullying Impacts The Reward Center Of The Brain


 Mirror
Mirror PrimeMind
PrimeMind Newswise
Newswise Science Daily
Science Daily